Life Sciences and Pharma Analytics That Speed Up Decisions
Cadeon helps life sciences and pharma teams connect research, manufacturing, quality, and commercial data into one clear view. Build reporting that teams trust, support regulated workflows, and move faster with better visibility across the full product lifecycle.

Quality and Deviation Analytics
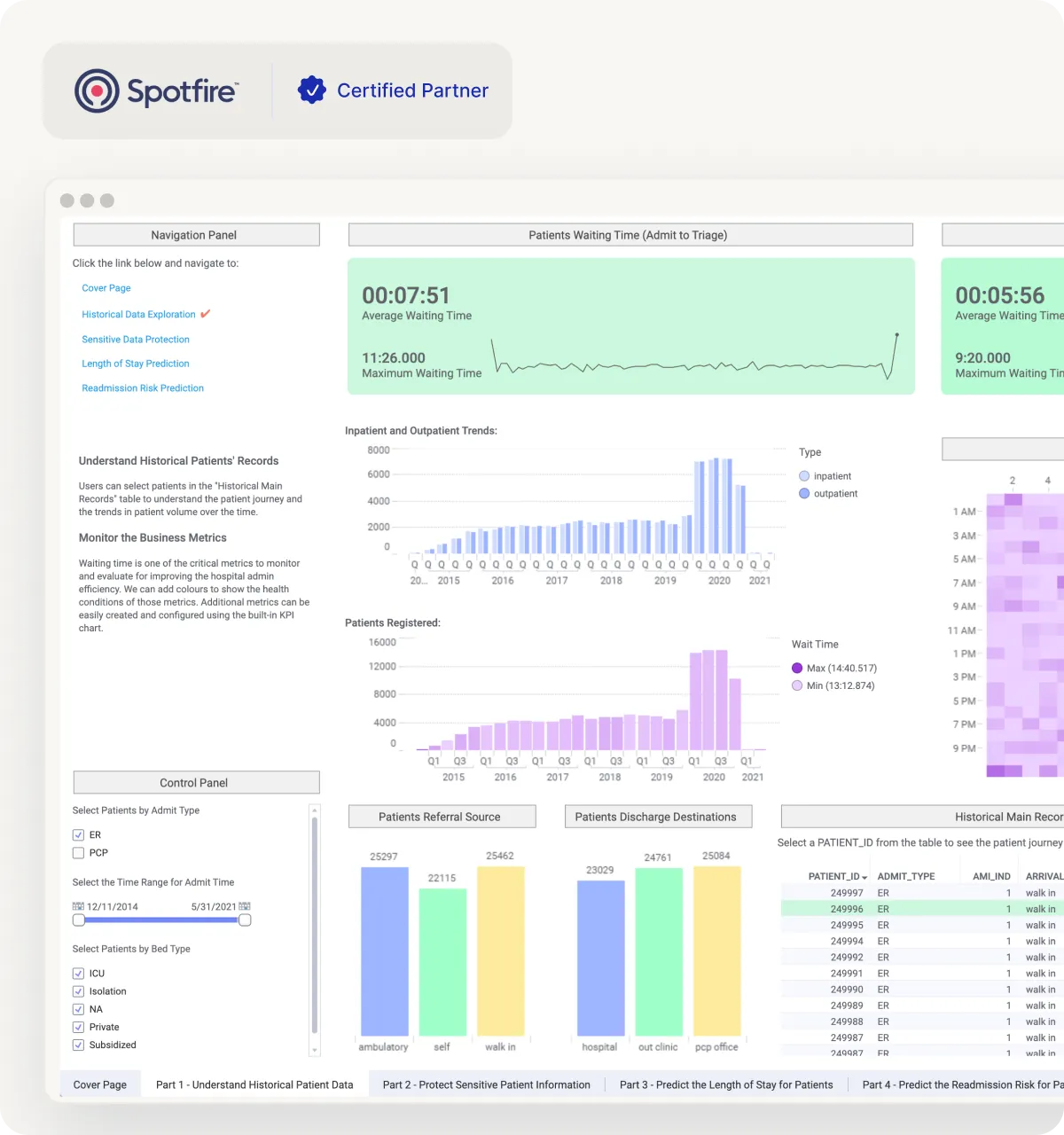
Track deviations, CAPA patterns, OOS events, and recurring issues with clear drilldowns.
Batch and Process Performance
Monitor batch cycle time, yield, and exceptions using connected manufacturing and lab data.

Compliance-Friendly Reporting
Standardize reporting logic and keep it traceable so audit prep becomes routine work.
Supply and Inventory Visibility
Connect planning and inventory signals to spot shortages early and reduce last-minute escalations.

Measured Results in Regulated Environments
Deviation, CAPA, and batch summaries update with fewer manual steps.

Standard KPI rules reduce conflicting numbers across teams and tools.
Issues surface earlier with connected lab, quality, and manufacturing views.
Built for regulated workflows
Reporting logic stays consistent and traceable across key metrics and teams.
Strong system connectivity
We connect lab, quality, manufacturing, and planning sources so data stops living in silos.
Dashboards teams actually use
Views are designed for daily checks, trend reviews, and structured reporting cycles.
Support that stays involved
We help with KPI changes, new views, and system updates as requirements shift.
Staying Clean After Go-Live
Processes change, products change, and reporting standards change. We keep your analytics stable so your team isn’t stuck fixing issues every week.
Monitoring and checks
We watch refresh cycles and key outputs so gaps get caught early.
Dashboards teams actually use
Views are designed for daily checks, trend reviews, and structured reporting cycles.
Add new views fast
Launch new dashboards or reports without rebuilding the whole setup.
Quality Signals Arrive Late or Out of Context
Deviations, OOS results, and CAPA data often sit separate from batch and production context. We connect the sources so teams can review trends with the “why” and “where” attached, not just the final number.
Too Many Versions of the Same KPI
Yield, cycle time, release timing, and quality counts can vary across reports. We standardize KPI logic and build one reporting layer so teams stop reconciling spreadsheets and start acting on the data.
Ready for Analytics That Holds Up Under Review?
Let’s connect your systems, standardize core KPIs, and build dashboards that support quality, compliance, and performance across life sciences and pharma.